Nitric oxide (NO) and carbon monoxide (CO) act as messenger molecules in the human body.[1] NO- and CO-releasing materials (NORMAs & CORMAs) are important for the development of safe gasotransmitter delivering devices for therapeutic purposes; toxic metabolites after gas release are kept in the biocompatible polymer matrix.[8] NO and CO photodonors use light as a convenient non-invasive on/off trigger since it allows the accurate control of site, timing and dosage.[2] Here we report the concept of embedding water-insoluble, photoactive NO and CO metal complexes into nanoparticles and fibrous polymer non-wovens.[3] NO and CO release into the surrounding medium is performed by light stimulation of the high surface area materials.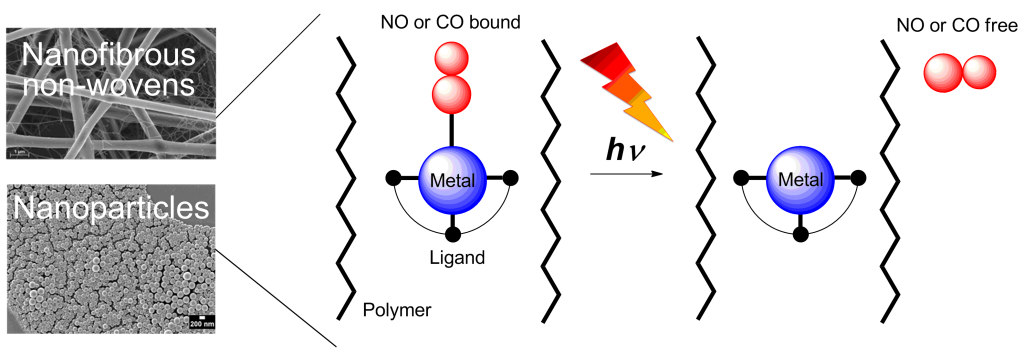 Metal nitrosyl or carbonyl complexes were non-covalently embedded into the polymer matrices via miniemulsion technique or electrospinning.[4,6,7] Especially dimanganese decacarbonyl in electrospun poly(L-lactide-co-D/L-lactide) fibers revealed nanoporous morphologies. A slight CO release from the metal complex induced nanoporosity in the electrospinning process.[5,7] Irradiation with light between 366 and 480 nm in water triggered NO/CO release from the nanoparticles or non-wovens.[4-7] With CORMA the cytotoxicity tests with 3T3 mouse fibroblast cells in the dark revealed a very low mortality rate. After illumination, CO bubbled out of the nanofibers thereby eradicating fibroblast cell cultures[7] or biofilms with methicilin-resistant Staphylococcus aureus (MRSA).[8]
Metal nitrosyl or carbonyl complexes were non-covalently embedded into the polymer matrices via miniemulsion technique or electrospinning.[4,6,7] Especially dimanganese decacarbonyl in electrospun poly(L-lactide-co-D/L-lactide) fibers revealed nanoporous morphologies. A slight CO release from the metal complex induced nanoporosity in the electrospinning process.[5,7] Irradiation with light between 366 and 480 nm in water triggered NO/CO release from the nanoparticles or non-wovens.[4-7] With CORMA the cytotoxicity tests with 3T3 mouse fibroblast cells in the dark revealed a very low mortality rate. After illumination, CO bubbled out of the nanofibers thereby eradicating fibroblast cell cultures[7] or biofilms with methicilin-resistant Staphylococcus aureus (MRSA).[8]
References
[1] S. H. Heinemann, T. Hoshi, M. Westerhausen, A. Schiller, Chem. Commun. 2014, 50, 3644-3660. [2] D. Crespy, K. Landfester, U. S. Schubert, A. Schiller, Chem. Commun. 2010, 46, 6651-6662. [3] A. Schiller, in Molecules at Work. Selfassembly, Nanomaterials, Molecular Machinery, (Ed.: B. Pignataro), Wiley-VCH, Weinheim, 2012, 315-338. [4] C. Bohlender, M. Wolfram, H. Görls, W. Imhof, R. Menzel, A. Baumgärtel, U.S. Schubert, U. Müller, M. Frigge, M. Schnabelrauch, R. Wyrwa, A. Schiller, J. Mater. Chem. 2012, 22, 8785-8792. [5] R. Wyrwa, M. Schnabelrauch, C. Altmann, A. Schiller, DE10 2012 004 132.2, PCT patent. [6] C. Bohlender, K. Landfester, D. Crespy, A. Schiller, Part. Part. Syst. Charact. 2013, 30, 138-142. [7] C. Bohlender, S. Gläser, M. Klein, J. Weisser, S. Thein, U. Neugebauer, J. Popp, R. Wyrwa, A. Schiller, J. Mater. Chem. B 2014, 2, 1454-1463. [8] DFG research unit FOR 1738, www.hhdp.uni-jena.de.